Cynthiana Research
Tissue Culture Improves the Propagation of 'Norton' Grapevine (Vitis aestivalis)
Brant B. Bigger and Paul E. Read
Department of Agronomy and Horticulture, University of Nebraska-Lincoln, Lincoln, Nebraska
Keywords: acclimation, in vitro, ex vitro, cytokinin, auxin, peat pellet, sundae cup
Abstract
Propagation of the Vitis aestivalis cultivar 'Norton' (syn='Cynthiana') through traditional woody cuttings has been difficult. Rooting of woody cuttings has been a major hindrance in propagating this cultivar and providing enough plants to meet grower needs. In vitro propagation offers another method of increasing plant material. Cultures were established and maintained on MS medium supplemented with 4 mM 6-benzyladenine (BA) and 0.5 mg•L-1 thiamine and solidified with 7.5 g•L-1 Bacto-Agar. The objectives of this study were to determine optimal methods for in vitro production and ex vitro establishment of 'Norton' plantlets. A factorial treatment with 4 concentrations of BA (0, 2, 4 & 8 mM) and 2 concentrations of naphthaleneacetic acid (NAA) (0 & 0.5 mM) was used for the multiplication study. Plantlets were acclimated to ex vitro conditions without in vitro rooting. Plantlets were rooted ex vitro either with or without a 1000 ppm (0.1%) indolbutric acid (IBA) dip for 5 seconds. Auxin did not have an effect on explant growth or plantlet rooting. Propagation of Norton through tissue culture appears to have potential to provide more plant material for growers in an expeditious manner.
Introduction
The American hybrid Vitis spp. 'Norton' is a premium wine grapevine for use in the central Midwest (Reisch et al., 1993). It has several desirable characteristics, but difficulty associated with propagation has limited its use in vineyards (Tarara and Hellman, 1991). Grapevines are traditionally propagated from cuttings of dormant one-year-old canes (Hartmann et al., 2002). Propagation of 'Norton' through this method has proven difficult because cuttings root poorly (Avery, 1999). In vitro propagation offers another method of increasing plant material for this cultivar. Micropropagation of Norton has previously been reported (Norton and Skirvin, 2001). The goal of this project is to improve in vitro plantlet quality and acclimate plantlets without an in vitro rooting step. Potential benefits of this research include increased plant material at a lower cost for growers and increase in availability of this grape for wineries.
Materials and Methods
Greenhouse-grown 3-year-old potted plants with actively growing shoots (30-50 cm in length) were used as source material. Axillary buds (0.5 X 0.5 cm) were excised, then surface disinfested for 15 minutes in a 10% commercial bleach solution and washed three times for 5 minutes in sterile water. Explants were placed in 25mm culture tubes containing 10 ml Murishige and Skoog (MS) medium supplemented with 4 mM 6-benzyladenine (BA) and 0.5 mg•L-1 thiamine and solidified with 7.5 g•L-1 Bacto-Agar. Established cultures were transferred monthly to fresh medium before experiments were begun. Explants were propagated placing two-node segments, with leaves removed, horizontally on the medium. Cultures were maintained at 23±1oC for 16 hours per day under cool white florescent light (28 mmo l• s-1 • m-2).
In vitro Propagation and Multiplication
MS medium supplemented with BA (0, 2, 4, or 8 mM), NAA (0 or 0.5 mM), 0.5 mg L-1 thiamine and solidified with 7.5 g•L-1 Bacto-Agar (pH 5.6±0.1) was used in all multiplication experiments. Explants were incubated as described above.
Ex vitro Acclimation
Four to six week old cultures with basal portions removed at approximately the level of the growth medium were used. Twenty-five plantlets with or without a five second dip in 1000 ppm (0.1%) indolbutric acid (IBA) were transferred to hydrated peat pellets within sundae cups with lids for rooting and acclimation . After four days, plantlets and peat pellets were planted in potting medium in 1 L plastic pots in the greenhouse under partial shade. Sundae cup lids were used to maintain plantlet humidity. Removing the lids gradually reduced humidity and after four weeks the plantlets were fully acclimated.
Experimental Design
Single explants placed in culture tubes were cultured as described above. Ten replications were assigned to each treatment in a factorial arrangement. The experiment was a completely randomized design and conducted twice. Data were collected after four weeks and statistically analyzed using SAS V8 GLM (SAS, 1999). Separation of treatment means was done by LSD at alpha 0.05 level.
Results and Discussion
Ninety-three cultures were initiated with a contamination rate of 4.3 % (4 of 93), and 61.3 % (57 of 93) of the cultures became fully established and growing in vitro.
Multiplication
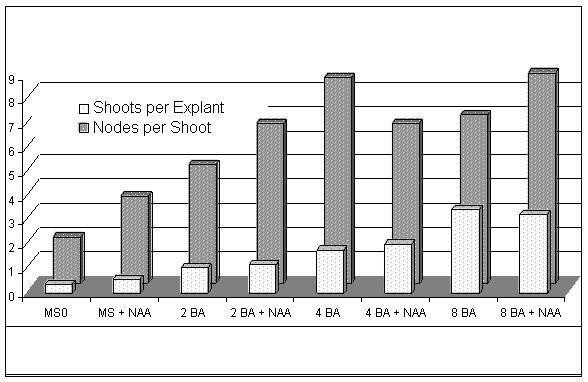
The effects of cytokinin, auxin, and interaction of cytokinin x auxin on number of shoots per explant and number of axillary buds per shoot are presented in Table 1. and Figure 1. Auxin did not have an effect on either the number of shoots per explant or the number of nodes per shoot. The interaction between cytokinin and auxin was not significant.
Acclimation
Fifty plantlets were transferred ex vitro for acclimation. After four weeks, 92% (23 of 25) and 88% (22 of 25) of the plantlets, respectively, survived and were growing vigorously. Plantlets rooted quickly and produced 3-7 primary roots (data not shown).
Conclusions
Tissue culture presents a method of efficient propagation of 'Norton' grapevine. Explants respond with more shoots and more nodes per shoot with increasing concentrations of cytokinin. High concentrations of cytokinin (8mM BA) produce plantlets of lower quality than lower concentrations (2 & 4 mM BA). Auxin does not play a significant role in explant growth or plantlet rooting at the concentrations tested. Producing acclimated plantlets is quick and efficient with simple rooting procedures. The in vitro rooting step is unnecessary. Although continued research is needed to evaluate field and grape characteristics of tissue culture derived 'Norton' grapevines, micropropagation has the potential to lower establishment costs for growers and increase grape availability for wineries.
Acknowledgements
Thanks to Virginia Miller, Mehmet Nuri Nas, and Sanjun Gu for advice and technical assistance.
Literature Cited
- Avery, J.D. 1999. Propagation of Norton-Cynthiana. Proc. of the 14th Annual Midwest Regional Grape and Wine Conf. p. 1-6.
- Hartmann, H.T., Kester, D.E. and Davies, F.T. Jr., 2002. Plant propagation: principles and practices. Prentice Hall, Edglewood Cliffs, NJ
- Norton, M.A. and Skirvin, R.M. 2001. Micropropagation of 'Norton' winegrape. HortTechnology. 11(2):206-208.
- Reisch, B.I., Goodman, R.N., Martens M.H. and Weeden N.F. 1993. The relationship between Norton and Cynthiana, red wine cultivars derived from Vitis aestivalis. Am. J. Enol. Vitic. 44(4):441-444.
- SAS, Inc. 1999. The SAS system for Windows, version 8.0. SAS Inst., Inc., Cary, N.C.
- Tarara, J.M. and Hellman, E.W. 1991. 'Norton' and 'Cynthiana'—premium native wine grapes. Fruit Var. J. 45(2):66-69.
Tables
|
Table 1. GLM for dependent variables: number of shoots/ explant and number of axillary buds/ shoot. |
|||||
|
|
|||||
|
|
|
number of shoots/explant |
axillary buds/ shoot |
||
|
Source |
DF |
F value |
F value |
||
|
BA |
3 |
63.96**** |
22.58**** |
||
|
NAA |
1 |
0.41 NS |
2.75 NS |
||
|
BA x NAA |
3 |
0.42 NS |
3.34** |
||
|
|
|||||
|
**, **** significant F-value at P<0.05 and 0.0005 level> NS nonsignificant at P>0.05 |
|||||
Figures
Figure 1. Effect of Cytokine and Auxin on the Number of Shoots per Explant and Nodes per Shoot